

home methods
FARMERS’ BULLETIN NO. 1474
U.S. DEPARTMENT OF AGRICULTURE
Washington, D. C.
Revised September 1942
Slightly revised April 1951
by
MARGARET S. FURRY, Assistant Textile Chemist
Off with stains! Keep clothes and home fabrics spotlessly clean. It is smart and thrifty to take best care of your clothing and household fabrics so they will look well and last as long as possible.
Many stained and spotted articles are needlessly thrown away each year. Many others are needlessly ruined by unsuccessful attempts to remove stains. It is possible to remove practically any stain at home by following a few simple rules. To take proper care of your fabrics, you will find it worthwhile to learn enough of the “know-how” skill so that you can do a good job.
One of the most important rules is to remove the stain promptly. Stains that become old usually require a remover so strong that it sometimes injures the cloth.
Another important rule is to select a remover that will not harm the cloth. If you can find out what caused the stain, this also will guide you in choosing the remover best suited for the job.
Work carefully, patiently, quickly. Often the way in which cleaning is done is as important as the kind of cleaning materials used, in getting good final results.
The following pages not only tell how to remove many kinds of stains but describe as well the general principles of stain removal.
First rule for success in removing a stain is to start while it is fresh, even before it dries if possible. Hot soapsuds or the heat of an iron sets some stains too, so that it takes strong treatment to loosen them. The professional cleaner always “spots-out” stains before he gives a garment a general cleaning or pressing.
Before starting to treat a stain, be sure you know what the cloth is made of—whether cotton, wool, silk, rayon, or a mixture. A stain remover successful on one kind of cloth may ruin another. Naturally, you want the method that will do the least possible damage to the cloth.
Strong acid removers destroy cotton and linen cloth; even mild acids, such as lemon juice and vinegar, may injure cotton and linen if allowed to remain too long on the cloth. If you use a mild acid to remove a stain, apply a weak alkali such as ammonia water or washing or baking soda immediately to stop the action of the acid. Wash the material in water after the treatment. (See p. 10.) Strong alkalies harm these materials also, but weak alkalies are safe to use if you rinse the article well in water afterwards.
All bleaches will rot cotton and linen if allowed to remain on the stain for more than a minute or two and will remove the color, too. Sodium perborate and hydrogen peroxide are the safest bleaches to use.
Strong acids and alkalies destroy wool or silk materials. Mild acids, except nitric, which weakens the material and turns it yellow, are safe to use. Even mild alkalies such as weak solutions of ammonia water, borax, or washing soda, must be used with care on wool. Bleaches that contain chlorine, such as ordinary bleaching powder, also destroy wool and silk. Sodium perborate is a good bleach to use, particularly on wool. Use lukewarm water—hot water turns both wool and silk yellow, shrinks wool, and injures the finish of silk.
Here are a few safety rules to follow in removing stains from rayon material. Never use strong acids or alkalies; they injure the material. Mild acids or alkalies usually do not harm it if properly rinsed. Water weakens rayon; do not pull or twist it when it is wet. Sodium perborate and hydrogen peroxide are the safest bleaches to use, but mild chlorine ones can be used with success.
Three kinds of rayon are made in this country—viscose, cuprammonium, and acetate. In removing stains from viscose and cuprammonium rayon, treat the material like cotton or linen. But acetate rayon is different. It dissolves in acetone, alcohol, or chloroform, so test a sample of any rayon material before using these liquids to remove a stain. Mixtures of alcohol and ether, or alcohol and benzene also are unsafe to use on acetate rayon or on colored material. Always mix alcohol with 2 or 3 parts of water before using it. Pressing with a hot iron may melt acetate rayon.
Synthetic materials, such as nylon and vinyon, are not harmed by either acids or alkalies. Water does not weaken them, as it does the rayon. They take up very little moisture, and as a result, stains such as coffee, tea, and fruit juice, remain on the surface and wash off easily. You may use bleaches safely on nylon or vinyon. But vinyon, like acetate rayon, dissolves in acetone and 3 chloroform, so test a sample of the material before using either of these to remove a stain. Press nylon with a warm (not hot) iron.
Other synthetic materials are made from peanut, corn, soybean, milk casein, and fish protein, but as yet they are not common and are not generally recognized. Treat them as you would silk and wool in removing stains.
Find out what the stain is, if possible, before trying to remove it. The wrong treatment may set a stain so that it is impossible to take it out. Always test water or any chemical stain remover on a sample of the cloth or on a hidden part of the garment (seam or hem) to be sure it will not change the color. You may have to choose between the stain and a faded spot.
If the stain is not greasy, first try to remove it with cold water. Hot water sets many stains and makes them harder to remove. Always test a sample of the cloth to see if water spots it. If not, place a pad of clean cloth underneath the stain, with the stain face down. To sponge, use a soft cloth, dampen it with cold water, and cover with a layer of dry cloth so that it is not too moist. Then sponge the stain with light, brushing motions, working from outside of stain to the center. Spread the moisture into the cloth around stain to keep a ring from forming.
The trick is to spread, or “feather out,” the liquid around the stain until there is no definite edge when the material dries. It may help to go over the spot with a cloth wet with alcohol mixed with 2 parts water. As alcohol changes some colors and dissolves acetate rayon, use it sparingly. Finally pat the spot with a dry cloth. Dry rapidly to prevent water rings.
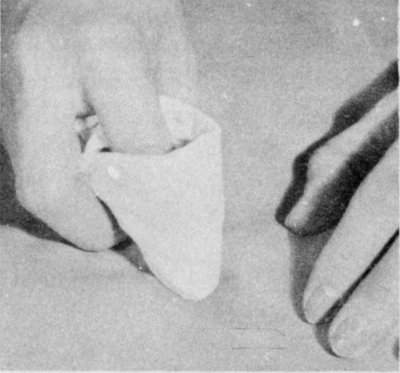
Sponge a nongreasy stain with water. Work from the outside of the stain to the center. Spread moisture unevenly into the cloth around the stain.
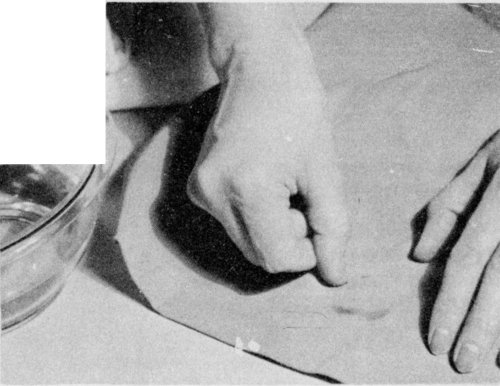
To remove a water ring, rub the cloth between the hands; then scratch with the fingernail.
If a ring has formed, remove it either by sponging the material with clean water or by shaking it in the steam from a briskly boiling teakettle. Scratching with the fingernail or a stiff brush or rubbing the cloth between the hands will sometimes remove a ring.
If a stain seems to be greasy, try a grease solvent, such as carbon tetrachloride, Stoddard solvent, gasoline, benzene, turpentine, ether, acetone, or alcohol. Most of these do not change the color of fabrics, but ether, acetone, and alcohol are apt to. So use them carefully on colored materials; always mix alcohol with 2 parts water. Either sponge the stain with the solvent or dip into a bowl of the liquid.
To sponge a grease spot, lay the stained material, wrong side up, on a pad of soft cloth. Apply the remover to the back of the cloth, so that the stain is washed from the material without having to pass through it. Sponge with a clean, soft, lintless cloth. Dip the cloth in the liquid and wring out most of the moisture. Sponge with light, brushing motions, working from the outside of the spot to the center. Work rapidly and use the solvent sparingly. It is better to apply the solvent several times quickly than to apply it once and leave it on for a long time.
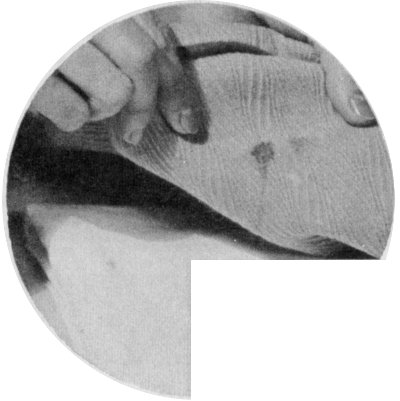
Sponge a greasy stain with carbon tetrachloride, gasoline, or benzene. Lay the stain face down on a pad of cloth.
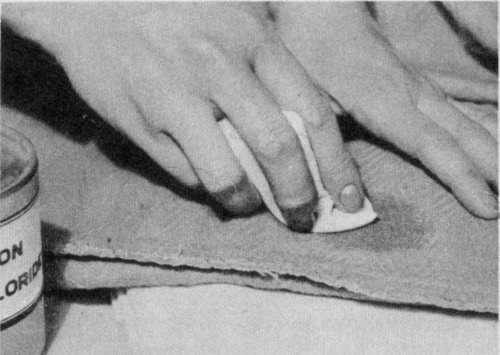
Use light brushing motions, working from the outside of spot to the center. Change the pad as it becomes soiled.
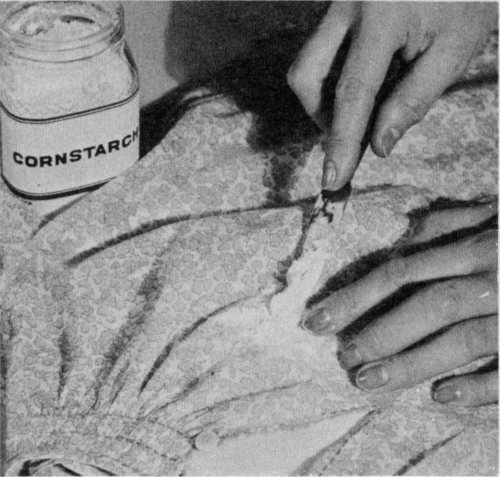
Sprinkle talcum, cornstarch, or chalk on a fresh grease or oil stain. Rub it in well, and let stand until it absorbs the grease; then brush off.
Avoid rings by spreading the cleaning fluid into the area around the stain and at the same time blowing lightly on the spot to dry it quickly. Do not rub—rubbing may cause light and worn-looking spots that are as bad as the stain. Change the pad as it becomes soiled. Finally pat the material with a dry cloth.
Always use these solvents out of doors or in a well-ventilated room, as it is harmful to breathe the vapors. Gasoline, naphtha, and ether catch fire easily and often explode, so never use them near a fire. Sometimes just rubbing a garment that is soaked with gasoline will cause it to burst into flames. Benzene, turpentine, alcohol, and acetone also are inflammable. For this reason, cleaning with large amounts of these fluids at home is not recommended. Grease-spot removers made entirely or in large part of carbon tetrachloride will not catch on fire.
Absorbent powders—chalk, talcum, corn meal, cornstarch—work well on light, freshly made stains such as grease spots or splatters of salad oil. Also such powders brush off readily and are safe to use on all materials. This method is not always successful, however, if the stain is very large or has become set or dry. To remove a stain with an absorbent powder, lay the stained article on a table and sprinkle a layer of the powder over the stain. Spread the powder around, and when it becomes gummy, shake or brush it off. Repeat this several times or until the stain disappears. If after several treatments the stain still shows, place the stain between clean blotting papers and apply a warm (not hot) iron for several minutes. Stains made by solid fats, such as butter, must be melted before the blotters can absorb them.
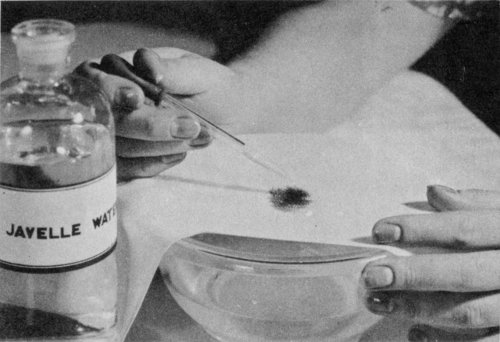
Use Javelle water to bleach stains from uncolored cotton or linen. Apply with a medicine dropper; rinse quickly in water. Apply a few drops of “hypo,” vinegar, or oxalic acid to stop the action of the chlorine.
Use bleaching chemicals carefully because most bleaches remove the color as well as the stain, besides weakening the cloth. Colored material in particular must be treated rapidly and rinsed well in water afterwards. Javelle water and other chlorine bleaches (sodium hypochlorite solutions) remove certain stains from uncolored cotton, linen, or rayon cloth. Do not use them on colored materials or on silk or wool.
To prepare Javelle water: Mix ½ pound washing soda in 1 quart of cold water. Add ¼ pound of bleaching powder (commonly called chloride of lime). Strain this liquid through a piece of muslin and store in a bottle with a tight cork or stopper ready for use.
To remove a stain with Javelle water, stretch the stained part of the cloth over a bowl filled with cold water and drop the Javelle water on the stain with a medicine dropper. (If the stain is large, dip the entire garment in the Javelle water). Never let the Javelle water remain on the stain for more than 1 minute; 7 it rots even linen and cotton materials if allowed to remain on them longer. Rinse quickly by dipping in the water.
Next apply a few drops of a solution made up of ½ teaspoon of sodium thiosulfate and 1 to 2 teaspoons of vinegar in 1 pint of water. This stops the action of the chlorine remaining in the cloth after the treatment with Javelle water. Then rinse the cloth well in clean water. You may use vinegar alone or oxalic acid solution (1 teaspoon oxalic acid to 1 pint water) instead of the thiosulfate solution, but they are not so satisfactory. To remove the stain completely you may have to repeat the Javelle water-thiosulfate treatment several times.
Sodium perborate is one of the safest bleaches for all types of materials. The treatment must be rapid and the sodium perborate well rinsed from the material, however, or it will take out the color. It will not remove some ink stains, iron rust, dyes and running color, or metal stains.
For small, fresh stains, sponge with a liquid made up of 4 tablespoons sodium perborate to 1 pint lukewarm water. Or stretch the stained cloth over a bowl of hot water, dampen the stain with water, and dust the powdered sodium perborate on it. Let stand a minute or two; then sponge or rinse well with water.
For a large stain, soak the entire garment for a half hour or longer in sodium perborate and soapsuds (4 tablespoons perborate to a pint of soapy water). To remove grass, beverage, mud, scorch, and some perfume stains, mix 1 level teaspoon sodium perborate with 1 pint hydrogen peroxide. But use this mixture immediately, as it soon loses its strength. Rinse in water.
Sodium perborate is particularly good to use on white woolens; it leaves them soft and fluffy.
Hydrogen peroxide, obtained at drug stores, is a good bleach for light scorch stains. The action of hydrogen peroxide is quicker if a few drops of ammonia water are added just before use. Or you can add 1 level teaspoon of either borax or sodium perborate to 1 pint of peroxide. Apply it to the stain with a medicine dropper, a glass rod, or sponge the stain with it. Follow by careful sponging or rinsing with water.
Oxalic acid is poisonous and should be handled carefully. Label it “Poison” and keep it out of the reach of children. Prepare a solution as follows: Dissolve about 3 tablespoons of the crystals of the acid in a pint of lukewarm water. Put in a bottle, stopper tightly, and use as needed. Stretch the stained cloth over a bowl of clean water and apply the oxalic acid to the stain with a medicine dropper or glass rod. Allow it to remain for a few minutes; then rinse quickly by dipping in the water. Apply a weak solution of ammonia water, borax, or sodium perborate to neutralize the action of the acid and rinse again. Never use oxalic acid on weighted silk.
Hydrosulfites are very useful to remove dye stains, iron rust, ink, mildew, grass, and fruit stains. Sodium hydrosulfite, the one most often used, may be bought at drug stores under many trade names as a dye or color remover for preparing cloth for redyeing. It should be stored in a tightly closed can so it will not become damp. To use, dissolve 2 teaspoons of the sodium hydrosulfite 8 in 1 pint of warm water and either sponge or dip the stained article in it. Or sponge the stain with water first, sprinkle the powder on the stain, and work it in well with the fingers. Rinse quickly. If used on colored material, hydrosulfites are apt to remove the color; so apply the treatment quickly and rinse well in water afterwards. Do not use on weighted silks.
Enzymes will remove certain stains from all kinds of materials. You can buy pepsin, the best known of the enzymes, at the drug store. It softens stains containing albumin (found in blood, gelatin, glues, certain medicines, eggs, milk, and ice cream), so that they wash out in water easily. Pepsin will soften these stains, even after they have been set by heat or alcohol. First be sure there is no soap or other alkali on the stain, or the enzyme will not react. Then dampen the stain with lukewarm water and sprinkle with pepsin powder. Let it stand for half an hour, keeping the spot damp. Or mix the pepsin with water (2 teaspoons to 1 pint lukewarm water) and sponge the stain with it. Sponge or rinse well with water.
Soaps and synthetic detergents (nonsoap cleaners) are helpful in removing grease and food spots, blood, and many other stains. For washing silks and woolens, select a mild soap. One with added alkaline salts may cause the colors to run and the cloth to become stiff and harsh. Also use a mild soap on all other delicate materials and on cotton, especially on those that are not guaranteed colorfast. If you are washing in hard water, add a water softener such as one of the special phosphates—sodium hexametaphosphate or tetrasodium pyrophosphate (sold under brand names)—which prevent the formation of hard-water scum. This scum is caused by the reaction of the soap and the calcium and magnesium compounds in the water. It settles on the clothes in gray or brown specks that are hard to remove.
Synthetic detergents come in powder, paste, and liquid form. Most of them suds and lather well, although a few clean without sudsing. They do not make a scum with hard water.
Synthetic detergents, like soaps, may be mild or alkaline. The mild synthetics are excellent for washing silks and fine fabrics, blankets, and sweaters. They are relatively safe for colors. The alkaline synthetics, which contain alkaline salts to aid in soil removal, are all-purpose washing agents for washing heavily soiled garments.
The nonalkaline detergents can be used in place of glycerine to loosen fresh tannin stains made by soft drinks and some fresh fruits. The other type should not be used because alkali tends to set tannin stains. Soap, even the mild type, is also alkaline enough to set these stains.
There are also special dry-cleaning soaps or benzene soaps, which, added to dry-cleaning fluid, aid in softening the stain and removing the dirt. Or, to soften a heavy grease or wax stain, put these soaps directly on the stain, especially on silk and wool cloth. Then rinse well in carbon tetrachloride, Stoddard solvent, gasoline, or benzene.
Keep all stain removers together on a handy shelf, but out of the reach of children. Label all the jars and bottles; be sure to mark “Poison” plainly on the poisonous ones. To have a complete shelf, you will need to keep at least three kinds of cleaning agents—bleaches, absorbent powders, grease solvents.
Act quickly when an acid has been spilled, for it may damage the cloth or destroy the color. First, wash the stain with cold water to stop the action of the acid. Rinse several times in cold water; then apply ammonia water or baking soda. Water alone will not restore color, but ammonia water may.
Baking soda.—Sprinkle soda on both sides of the stain, moisten with water, and allow to stand until the bubbling stops. Rinse well with water.
Ammonia water.—Hold the dampened stain over an open bottle of strong ammonia water; or if the material doesn’t water-spot, put a few drops of ammonia water, diluted to half strength on the stain. Since ammonia water affects some dyes, have white vinegar ready to apply quickly if the color changes. Rinse well with water.
Sponge or soak the stain in carbon tetrachloride, benzene, or kerosene. Kerosene will make the cloth oily, so wash in warm suds after the treatment.
Alcoholic beverages and soft drinks may cause tannin stains. Fresh tannin stains are almost colorless, but if they are allowed to stand or are washed in soap and water or heated as in ironing and pressing, they turn brown and are almost impossible to remove. Fresh stains can be removed as follows:
Cold water and glycerine.—Sponge the stain with water or with a mixture of equal parts alcohol and water. Then pour glycerine on the stain and rub between the hands. Let stand for a half hour and rinse with water.
Acetic acid.—If the above treatment does not remove the stain, apply a 10-percent solution of acetic acid with a medicine dropper and let stand a few seconds. Rinse and repeat if necessary. Stop the action of the acid with baking soda or ammonia (see above) and spread the garment in the sun.
Bleaches.—The last traces of stains on white materials can sometimes be removed by bleaching. Use one of the following:
Hydrogen peroxide or sodium perborate.—Sponge lightly with hydrogen peroxide or with a mixture of 1 level teaspoon sodium perborate to 1 pint hydrogen peroxide. If this does not remove stain, cover dampened spot with powdered sodium perborate and let stand an hour. Rinse in water.
Javelle water.—For stains on uncolored cotton or linen material, dip in Javelle water for 1 minute (no longer), remove the chlorine from the cloth with a sodium thiosulfate solution, rinse well in water. (See p. 6 for more detailed instructions.) Do not use Javelle water to remove stains from colored materials or from silk or wool.
Remove alkali spots at once; they may destroy not only the color but the material as well. First sponge or rinse the spot thoroughly with cold water. This generally is sufficient for mild alkalies such as washing soda and weak ammonia water. But to be on the safe side and to help restore color, apply an acid—this stops the action of the alkali. Then rinse or sponge the spot thoroughly with water. Use any of the following mild acids:
Lemon juice.—Squeeze the juice on the stain, and allow it to remain until the juice loses its bright yellow color. Sponge or rinse well with water.
Vinegar.—Sponge with vinegar; then rinse in cold water.
Acetic acid.—Apply a few drops of a 10-percent solution of acetic acid with a medicine dropper or a glass rod and remove the excess by rinsing or sponging with water.
Argyrol stains must be treated while still fresh. Proceed as follows:
Pepsin.—Sponge with warm water to remove any argyrol that has not soaked into the cloth. Then sprinkle powdered pepsin over the dampened stain. Work it well into the cloth, let stand a half hour or longer, then sponge with water.
Iodine and sodium thiosulfate (“hypo”).—After the above treatment, put a few drops of tincture of iodine on the dampened stain with a glass rod. Let stand for 10 or 15 minutes; then sponge with a solution prepared by dissolving several crystals of sodium thiosulfate in ½ cup of water. Rinse well in water.
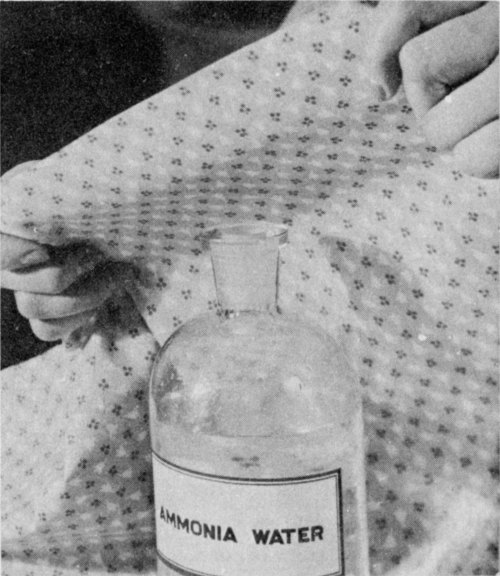
To bring back the color changed by an acid, hold the dampened stain in the fumes from an open bottle of ammonia water.
Blood stains will usually come out if sponged or washed in cold or lukewarm water first. Never use hot water; it sets the stain.
Cold or lukewarm water.—For stains on silk or wool, sponge with cold or lukewarm water. For washable material, soak the stains in cold water until they turn light brown in color; then wash in warm soapy water. If the stain is an old one and has dried, it may help to add 2 tablespoons of ammonia water to each gallon of water used for soaking. Strong salt water (about 2 cups of salt to 1 gallon water) is also good to loosen the stain.
Hydrogen peroxide and sodium perborate.—If the above treatment does not completely remove the stain, sponge with hydrogen peroxide. Or sponge with a mixture of 1 level teaspoon sodium perborate to 1 pint hydrogen peroxide. If the stain still shows, cover the dampened spot with powdered sodium perborate and let stand an hour. Rinse thoroughly. These bleaches will not harm the cloth, but before using test for colorfastness on a sample of cloth or on an inner seam of the garment. If the color fades, do not use the bleaches; just dampen the stain and spread in the sun to bleach.
Starch.—Use a starch paste to remove stains on thick materials, such as flannel and blankets, which cannot be soaked in water. Mix raw starch to a paste with cold water, apply the paste thickly to the stain, and brush it away when it dries. Repeat the treatment until the stain disappears.
Soak fresh stains or rinse them in cold water. Or wash the stained article with plenty of warm soapy water, rubbing thoroughly.
Scrape away as much wax as possible with a dull knife. Then treat as follows:
Blotting paper.—Place the stain between clean white blotters, cleansing tissues, or paper towels, and press with a warm iron, changing the blotters as they become soiled. Then sponge with carbon tetrachloride or other grease solvent.
Denatured alcohol.—If a color stain remains, sponge with liquid made up of 1 cup denatured alcohol and 2 cups water.
Launder in warm soapy water if the material is washable. Otherwise, sponge with clear warm water.
If dye or chocolate stains remain, follow instructions given under Dyes and Running Colors, page 14, or Chocolate and Cocoa, page 13.
Carbon-paper stains usually can be removed by washing in a heavy suds of soap and water. Sponge unwashable materials with a liquid of 1 cup alcohol and 2 cups water; then sponge with cold water.
Use one of the following methods:
Ice.—If the material will not water-spot, rub the gum stain with ice. Then scrape and rub the hardened gum out of the cloth. This method is particularly good for rugs and other heavy materials.
Egg white.—If the material is washable, soften the gum stain with egg white and then wash.
Carbon tetrachloride, kerosene, or turpentine.—Soak the stain in carbon tetrachloride, kerosene, or turpentine. If kerosene is used, wash in warm soapy water afterwards.
It may be necessary to try more than one method to remove chocolate and cocoa stains, since they usually contain other substances such as fat, milk, starch, and sugar. First scrape off as much of the stain as possible with a dull knife; then try one of the following:
Soap and warm water.—If the material is washable the regular laundering in warm soapy water will often remove this stain.
Hydrogen peroxide and sodium perborate.—Sponge stubborn stains with hydrogen peroxide. Or use a mixture of 1 level teaspoon sodium perborate to 1 pint hydrogen peroxide. If the stain still shows, cover the dampened spot with powdered sodium perborate and let stand an hour. Rinse thoroughly. Be sure to test for color change on a sample of the cloth or on the inside of hem or seam of the garment before using these bleaches on the stain.
Carbon tetrachloride and pepsin.—If the cloth is not washable sponge with carbon tetrachloride to dissolve the grease. Dry thoroughly, then sponge with warm water, and dust with pepsin powder. Work the powder into the cloth, let stand for 30 minutes or longer, then sponge with water.
Fresh cod-liver oil stains are almost colorless and are easy to remove. But old stains, especially if the material has been washed or ironed, are a light brown and are almost impossible to remove, even with bleaches. Treat fresh stains with either of the following:
Grease solvents.—Sponge or dip fresh cod-liver oil stains in carbon tetrachloride, benzene, Stoddard solvent, or gasoline. When the cod-liver oil has been removed, sponge with warm soapy water.
Glycerine.—For washable materials, pour either glycerine or one of the soapless shampoos on the fresh stain. Rub lightly between the hands to loosen the stain, rinse well in water, and then wash in warm soapsuds.
Water and glycerine.—If the stains are on wool or silk, sponge with lukewarm water. Then apply glycerine and rub lightly between the hands. Let stand for half an hour and rinse thoroughly with water. If a grease spot from cream remains, sponge with carbon tetrachloride.
Boiling water.—Remove fresh stains from washable materials by pouring boiling water on the stain from a height of 2 or 3 feet, then wash in warm soapy water. If a trace of stain remains, dry in the sun or bleach with hydrogen peroxide and sodium perborate.
Hydrogen peroxide and sodium perborate.—Sponge with clear water and then with a solution of 1 teaspoon sodium perborate to 1 pint hydrogen peroxide. If the stain still shows, sprinkle powdered sodium perborate on the stain and let stand half an hour. Rinse well with water.
As there are many different kinds of dyes, no one remover will successfully take out all dye stains. In fact, it may be impossible to remove some of these stains completely. Proceed as follows:
Water and sunlight.—If the material is washable, rinse the stains in cold or lukewarm water (soak for 10 to 12 hours if necessary), wash in heavy soapsuds, and then dry in the sun. Spots on wool or silk materials sometimes come out by soaking or washing in cold water.
Bleaches.—If a stain remains, try one of the following:
Hydrosulfite.—Apply one of the hydrosulfites available at drug stores as a color remover. Follow directions on the package.
Javelle water.—For stains on uncolored linen, cotton, or rayon, dip in Javelle water for 1 minute (no longer), remove the chlorine from the cloth with a sodium thiosulfate solution, rinse well in water. (See p. 6 for more detailed instructions.) Do not use Javelle water to remove stains from silk or wool.
Hydrogen peroxide.—For stains on any white material, add a few drops of ammonia water to hydrogen peroxide. Soak the stains until they disappear and rinse thoroughly in water. One teaspoon sodium perborate added to 1 pint hydrogen peroxide makes a good bleach, but it must be made fresh, as it soon loses its strength.
Scrape away as much of the stain as possible with a blunt knife. Then sponge with cold water. Never use hot water—heat makes egg stains harder to remove.
Pepsin.—If cold water does not remove the stain completely, sprinkle pepsin powder over the spot. Work it in well and let stand for half an hour. Rinse well.
Grease solvents.—For nonwashable materials, sponge first with cold water. Let dry and then sponge with carbon tetrachloride, gasoline, or other grease solvent.
Acetone or nail-polish removers.—On any material except acetate rayon or vinyon, sponge the stain with acetone or a commercial nail-polish remover.
Grease solvent and banana oil (amyl acetate).—Use this treatment on any material including acetate rayon and vinyon. First wet the stain well with carbon tetrachloride or gasoline; then apply a drop of banana oil to the stain. Brush lightly with a soft cloth, using an upward motion to pick up the dissolved polish. For heavy stains use dry-cleaning soap with the banana oil.
Bleaches.—To remove any color remaining after the polish itself has been dissolved, apply a bleach. Test the cloth for change in color first.
Hydrogen peroxide and sodium perborate.—Sponge with clear water and then with a solution of 1 teaspoon sodium perborate to 1 pint hydrogen peroxide. If the stain still shows, sprinkle powdered sodium perborate on the stain and let stand half an hour. Rinse well with clear water.
Hydrosulfite.—Apply one of the hydrosulfites available at drug stores as a color remover. Follow directions on the package.
Soak or sponge the stain with a solution made of ½ cup salt and 1 cup vinegar in 2 quarts of water. Rinse well in water; then wash in warm soapsuds.
Sponge the stain with carbon tetrachloride or benzene. If the material is washable, soak in kerosene and then wash in warm soapy water.
Treat fruit and berry stains immediately, if possible; they are hard to remove after they dry. Boiling water (if it does not harm the cloth) or sometimes even warm water will remove most fruit stains. It is better not to use soap, as alkalies set some fruit and berry stains. Use the same methods for removing stains from cooked fruits and berries as from fresh.
Washing in warm soapy water sometimes removes stains from citrus fruits, such as grapefruit and lemon. But if the stain is old or the cloth has been pressed before washing, use one of the bleaches described below. If the acid in citrus fruit changes the color of the cloth, restore it with ammonia water or baking soda. (See Acids, p. 10.)
Cold water and glycerine.—For fresh peach, pear, cherry, and plum stains on cotton and linen and for any fruit stain on wool or silk materials (either white or colored), first sponge the stain well with cool water; then work glycerine or a soapless shampoo into the stain, rubbing lightly between the hands. Do not 16 use soap, as soap sets the stain. Let stand several hours, then apply a few drops of vinegar or oxalic acid, allow to remain for a minute or two, then rinse thoroughly in water.
Boiling water.—Boiling water removes from cotton and linen most fruit stains except peach, pear, plum, and cherry. Never use boiling water on silk or wool. Stretch the stained part over a bowl, fasten it with string, and pour boiling water on it from a teakettle held at a height of 3 or 4 feet so that the water strikes the stain with force. Rubbing alternated with the boiling water is also helpful. If a stain remains, squeeze a little lemon juice on it and place in the sun to dry, or use one of the chemical bleaches.
Bleaches.—If a stain remains, try one of the following:
Hydrogen peroxide and sodium perborate.—Sponge with hydrogen peroxide-sodium perborate mixture (1 teaspoon sodium perborate to 1 pint peroxide). Rinse thoroughly. If the stain persists, sprinkle powdered sodium perborate on the dampened area and let stand for half an hour. Finally rinse well. Always test for change of color on the inside of a hem or seam before using these bleaches. If the color fades, do not use them—just dampen the stain with water and spread in the sun to bleach.
Hydrosulfite.—Hydrosulfites available at drug stores as dye removers are satisfactory for removing fruit stains from any white material. Follow directions on package.
Javelle water.—For stains on uncolored linen or cotton material, dip in Javelle water for 1 minute (no longer), remove the chlorine from the cloth with a sodium thiosulfate solution, rinse well in water. (See p. 6 for more detailed instructions.) Do not use Javelle water on silk or wool.
Water.—If the material is washable, soak the spot in warm water, or if it is a stubborn stain you may have to boil it. If the stain is known to be casein glue, soak it in cold water.
Acetic acid.—For nonwashable materials, sponge the spot with water, then with acetic acid (10-percent solution) or white vinegar. Rinse well.
Hot water and soap.—If the material is washable, use hot water and soap, rubbing the stain well. If this does not completely remove the stain, use a bleach.
Bleaches.—Try one of the following:
Javelle water.—For stains on uncolored linen, cotton, or rayon, dip in Javelle water for 1 minute (no longer), remove the chlorine from the cloth with a sodium thiosulfate solution, rinse well in water. (See p. 6 for more detailed instructions.) Do not use Javelle water to remove stains from silk or wool.
Hydrogen peroxide and sodium perborate.—Sponge with clear water and then with a solution of 1 teaspoon sodium perborate to 1 pint hydrogen peroxide. If the stain still shows, sprinkle powdered sodium perborate on the stain and let stand half an hour. Rinse well with clear water. Always test for change of color on a sample of the cloth before using these bleaches.
Hydrosulfite.—Hydrosulfites available at drug stores as dye removers are satisfactory in removing grass stains from any white materials. Follow directions on the package.
Benzene or denatured alcohol.—On materials that soap and water might injure, sponge the stains with benzene or alcohol. Test them first to be sure they do not change the color of the material. Do not use alcohol on acetate rayon or vinyon unless you dilute it—1 cup denatured alcohol with 2 cups water.
Fresh grease spots usually are the pure fat or oil. Old grease spots or stains from automobile, wheel, or machine greases usually contain also more or less dust, dirt, or fine bits of metal. (For road oil and axle grease, see p. 27.) First scrape or wipe off as much of the grease as possible; then treat the stain by one of the following methods:
Soap and water.—If the material is washable, wash in warm sudsy water. Be sure to use plenty of soap on the stained part and rub well between the hands. The soaplike washing agents (soapless shampoos, oils, and lathers) are good to soften grease stains.
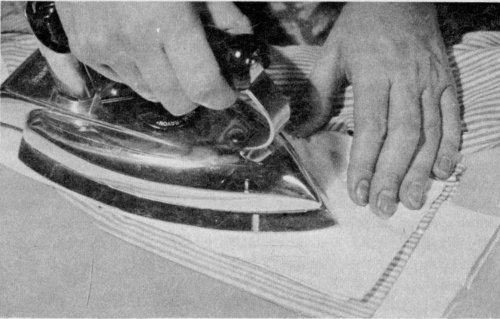
Place a grease or oil stain between paper towels or cleansing tissues and press with a warm iron.
Absorbents.—Use cornstarch, French chalk, or white talcum powder for fine materials; corn meal or salt for carpets, rugs, and other coarse materials. 18 Dust the powder or salt over the spot, let stand until it absorbs the grease or oil, then brush off. Another method is to place the stained part between blotting papers and press lightly with a warm iron. Change the blotting paper as it becomes soiled. Or use cleansing tissues or paper towels in the same way. The advantage of using absorbents is that they do not wet the material or leave rings as water or grease solvents are apt to do.
Grease solvents.—Remove common grease and oils with carbon tetrachloride, gasoline, or benzene. Place a pad of clean cloth or a white blotter beneath the stain and sponge with a clean cloth, moistened with the grease solvent. Work from the wrong side of the material in order to push the dirt and grease out rather than to rub it into the material. Use light, brushing motions, work from the outside of the spot toward the center and spread or “feather out” the solvent into the cloth around the stain until there is no definite edge. Then pat dry with a clean, dry cloth.
If the grease spot contains dirt or fine bits of metal, first loosen the stain by rubbing a little lard, petroleum jelly, or dry-cleaning soap into it. Then sponge with the grease solvent or dip the stain into a small bowl of the solvent.
Another method is to make a paste by mixing cornstarch or talcum with carbon tetrachloride or other dry-cleaning fluid. Spread the paste over the spot; when dry brush it off. Repeat if necessary. The solvent does not spread and is less likely to form a ring if used in this way.
Ice cream stains contain milk or cream, sugar, sometimes egg, and often coloring. If after trying the following methods, a fruit or chocolate stain remains, follow instructions under Fruits and Berries, page 15, or under Chocolate and Cocoa, page 13.
Cold or lukewarm water.—If the material is washable and the stain contains no highly colored fruit or chocolate, sponge with cold or lukewarm water; then wash in warm soapsuds.
Carbon tetrachloride.—For nonwashable materials, sponge with carbon tetrachloride to remove the greasy part of the stain. Let it dry; then sponge with cold water to remove any stains from the egg and sugar in the ice cream. If this does not remove the stain completely, follow with a pepsin treatment.
Pepsin.—First sponge the stain with cold water, then sprinkle pepsin on the dampened stain, and let it stand half an hour. Brush it off and rinse the spot well. For best results, be sure the material is free from soap or other alkali before applying the pepsin.
Because inks differ in composition, it is impossible to find removers that are equally effective for all types of ink spots. Each of the methods mentioned below is satisfactory with some type of ink. For most ink spots, it is necessary to try several methods, beginning always with the simplest and that least likely to harm the cloth.
Denatured alcohol, carbon tetrachloride, and benzene.—Place a pad of cloth or blotter under the stain and sponge with one of these solvents. Then rub glycerine (use glycerine only with alcohol) or a dry-cleaning soap into the stain and finally rinse out with the solvent. If this does not remove the stain, let the stain dry; then wet with water and rub in a synthetic detergent (nonsoap cleaner) to help soften the stain. Or use strong soapsuds to which a few drops of ammonia water have been added. Alcohol must not be used on acetate rayon or colored materials.
Use one of the following agents for removing printing-ink stains:
Lard or petroleum jelly.—Rub the stain with lard or petroleum jelly; work it into the cloth. If material is washable, wash with soap and water; otherwise sponge with carbon tetrachloride, gasoline, or other grease solvent.
Turpentine.—Soak the stain for a few minutes in turpentine and then sponge with carbon tetrachloride, alcohol, or other dry-cleaning fluid. Do not use alcohol on acetate rayon or colored materials.
Kerosene.—To remove printing from flour bags and other bags, soak in kerosene for several hours. Then wash thoroughly in soap and hot water and spread on the grass in the sun to dry.
In removing writing-ink stains it usually is necessary to try various methods. Always start with the simplest method and the one least likely to harm the cloth.
Absorbents.—If the stain is still wet, spread corn meal, salt, French chalk, cornstarch, or talcum powder on the stain to remove any excess ink and to keep it from spreading. Work the powder into the stain. Shake it off as it becomes soiled and repeat the process. When the dry absorbent fails to take up more ink, make the absorbent into a paste with water or with a mixture of 1 part water and 1 part alcohol and apply again. Let dry and brush off.
Glycerine and water or soap and water.—If the material is washable, pour either glycerine or one of the soapless shampoos on the fresh stain. Rub lightly between the hands, rinse, and apply glycerine again as long as any ink comes from the stain. Rinse with clear water. Washing with soap and warm water will remove some types of ink.
Bleaches.—If the above treatments do not remove the stain, try a bleach. But use bleaches sparingly on colored materials.
Oxalic acid.—Soak the stain for a few seconds in a solution of oxalic acid (3 tablespoons of the crystals of the acid to a pint of water). Or sponge the stain well with cold water, then stretch the stain over a bowl of hot water, and apply crystals of oxalic acid directly to the stain. Rinse by dipping in the hot water and finally in water to which a few drops of ammonia water have been added. Do not use on weighted silk.
Hydrosulfite.—Sponge with a hydrosulfite solution and rinse quickly.
Soap and water.—If the material is washable, soap and water will often remove a fresh stain. Or moisten with water and place either in the sun, over a warm radiator, or hold in the steam from a boiling teakettle.
Denatured alcohol.—On materials that water would injure, sponge with alcohol. On acetate rayon and colored materials be sure to dilute the alcohol—1 cup denatured alcohol to 2 cups water.
Sodium thiosulfate (“hypo”).—Sponge the stain or dip in a solution of 1 tablespoon of the “hypo” to 1 pint of water. Rinse well in water.
Use any of the methods given below to remove iron-rust stains from white materials. Test remover on sample of cloth before using on colored materials.
Lemon juice.—Spread the stain over a pan of boiling water and then squeeze lemon juice on it. After a few minutes rinse; then repeat the process. This method is rather slow, but does not harm delicate white cottons or linens. Another method is to sprinkle the stain with salt, squeeze lemon juice on it, and spread in the sun to dry. Add more lemon juice if the stain still shows. Rinse well.
Oxalic acid.—Spread the stained article over a bowl of hot water and apply a few drops of oxalic acid solution (3 tablespoons of the crystals to 1 pint of water). Or put the crystals of acid directly on the stain and moisten with hot water. Rinse in hot water, and repeat until the stain disappears. Do not use on weighted silk.
Cream of tartar.—Boil the stained article in a liquid made up of 4 teaspoons of cream of tartar to 1 pint of water. Rinse thoroughly.
Hydrosulfite.—Hydrosulfites available at drug stores as color removers or dye-stripping agents also will remove rust stains. Follow directions given on the package. Do not use on weighted silks.
Sponge with carbon tetrachloride or benzene. Treat as for cod-liver oil stains.
Petroleum jelly and carbon tetrachloride.—If water spots the cloth, work petroleum jelly or lard into the stain. Then either sponge with carbon tetrachloride or dip the stained part in a bowl of the solvent. If a trace of color remains, sponge with denatured alcohol. On acetate rayon and colored materials, dilute the alcohol—1 cup of denatured alcohol to 2 cups water.
Glycerine, soap, and water.—If the material is washable, first loosen the stain as above with glycerine or petroleum jelly. Then launder. If soap or other alkalies are applied before the stain is loosened, they are apt to set it.
Hydrogen peroxide and sodium perborate.—Sponge with sodium perborate-hydrogen peroxide mixture (1 teaspoon sodium perborate to 1 pint peroxide). Rinse thoroughly. If the stain persists, sprinkle powdered sodium perborate on the dampened area and let stand for half an hour. Finally rinse well. Be sure to test the cloth for colorfastness before using these bleaches.
Sponge meat-juice or gravy stains with cold or lukewarm water. Never use hot water; it sets the stain. If a grease spot remains, launder washable materials in warm soapy water. If the cloth is not washable, use an absorbent powder or a grease solvent.
Absorbents.—Dust the powder over the stain, let it stand until it absorbs the grease, then brush off.
Solvents.—Sponge with carbon tetrachloride, gasoline, or benzene.
Because of the great number and variety of substances used in medicines, it is not possible to give methods for removing all such stains. If you know what the medicine is made of, it will aid in choosing the remover. For instance, a tarry or gummy medicine can be treated in the same way as a tar spot (see p. 27); a medicine containing much iron can be removed in the same way as iron rust (see p. 20). Medicines in a sugar sirup usually can be washed out with water; those dissolved in alcohol sometimes can be removed by sponging the stain with alcohol. Many of the medicines used in swabbing sore throats contain silver nitrate and should be sponged with a solution of sodium thiosulfate (“hypo”)—1 teaspoon of the crystals in 1 cup of water.
If you cannot find out what kind of medicine caused the stain, you may have to try several methods to find one that will do the job. Each of the following methods will remove certain medicine stains.
Boiling water.—For washable materials, pour boiling water on the stain from a height of 3 or 4 feet, as for fruit stains, or launder in warm soapy water.
Denatured alcohol or carbon tetrachloride.—Some color stains can be sponged or soaked out with alcohol. Sponge greasy stains with carbon tetrachloride. A dry-cleaning soap helps to loosen them. Finally sponge with fresh carbon tetrachloride.
Bleaches.—Use bleaches only on white materials. Try one of the following:
Hydrosulfite.—Use one of the hydrosulfite dye-stripping agents available at drug stores. Follow instructions on the package.
Javelle water.—For stains on linen, cotton, or rayon, dip in Javelle water for 1 minute (no longer), remove the chlorine from the cloth with a sodium thiosulfate solution, rinse well in water. (See p. 6 for more detailed instructions.) Do not use Javelle water on silk or wool.
Mercurochrome stains are very hard to remove unless you treat them promptly. Proceed as follows:
Denatured alcohol, glycerine, and laundering.—First sponge the stain well with a liquid made of equal parts of alcohol and water. (On acetate rayon and colored materials use 1 part alcohol and 2 parts water). Next work glycerine into the cloth to help loosen the stain, and continue using as long as any color bleeds from the stain. Then wash well in soapsuds, and rinse with water to which a few drops of ammonia water have been added.
Acetic acid.—If a stain remains after the above treatment, apply 10-percent acetic acid with a medicine dropper; then rinse well in water.
Bleaches.—If the above treatments do not completely remove the stain, use a bleach.
Javelle water.—For stains on uncolored linen, cotton, or rayon, dip in Javelle water for 1 minute (no longer), remove the chlorine from the cloth with a sodium thiosulfate solution, rinse well in water. (See p. 6 for more detailed instructions.) Do not use Javelle water on silk or wool.
Sodium perborate.—Sponge with a sodium perborate solution (4 tablespoons of the perborate in a pint of lukewarm water) or dampen the stain with water and dust the powdered sodium perborate on it. Rinse thoroughly. Always test for the effect on the color of the cloth before using bleaches.
Sodium hydrosulfite.—This color remover is available at drug stores. It may be used safely on most white materials. Follow directions on the package.
The tarnish of copper, brass, tin, and other metals often stains textiles. To remove, apply vinegar, lemon juice, or a 10-percent solution of acetic acid. Rinse well as soon as the stain has dissolved. Do not use chlorine bleaches or sodium perborate to remove these stains.
Mercury or quicksilver removes lead or solder stains from rugs or clothing. First scrape off as much of the lead as possible with a dull knife. Then pour mercury on the stain and work with a stick until the mercury absorbs the stains.
Mildew spots must be treated when fresh, before the mold growth has a chance to weaken the cloth.
Soap and water.—On washable material, soap and water will remove very fresh stains. Drying on the grass in the sun helps to bleach the spots.
Bleaches.—Try a bleaching agent if soap and water do not remove the stain. Be sure to test for colorfastness on a hidden part of the garment.
Lemon juice.—Moisten the stain with lemon juice and salt and place in the sun. This often removes slight stains.
Javelle water.—Old stains on cotton, linen, or rayon may be bleached out with Javelle water. Dip the stain in the Javelle water for 1 minute (no longer), remove the chlorine from the cloth with a sodium thiosulphate solution, rinse well in water. (See p. 6 for more detailed instructions.) Do not use Javelle water on silk or wool.
Sodium perborate.—Soak the stain in a sodium perborate solution (4 tablespoons perborate to 1 pint lukewarm water). Or dampen the stain with water and sprinkle the perborate powder directly on the stain. Rinse after either treatment.
See Ice Cream, p. 18.
Follow instructions given under Fingernail Polish, page 15.
Soak in lukewarm salt water (about 2 cups salt to 1 gallon water) or in weak ammonia water (2 tablespoons ammonia water to each gallon water). Rinse well with cold water and launder as usual.
Let the mud stain dry, then brush well. Sponge with clear water, or use soap and water if it will not harm the cloth. Sponging with alcohol will help to remove the last traces of the stain. On colored materials and acetate rayon dilute the alcohol—1 cup denatured alcohol to 2 cups water.
Glycerine and soap and water.—If the material is washable, work glycerine into the stain, rub lightly between the hands, and then wash the article in soap and water.
Denatured alcohol.—If water spots the cloth, sponge the stain with alcohol. Since alcohol makes some colors run, test a sample of the cloth to be sure it does not harm the color. On acetate rayon sponge with dilute alcohol—1 cup denatured alcohol to 2 cups water.
Bleaches.—Try one of the following, but use sparingly on colored materials and do not use on weighted silks.
Hydrosulfite.—Sponge with a hydrosulfite solution (2 teaspoons in 1 pint of warm water) and rinse quickly.
Oxalic acid.—Apply oxalic acid solution with a medicine dropper (see p. 7) and rinse well with clear water. Sponge with weak ammonia water, borax or sodium perborate solution, to neutralize the acid.
Treat oil paint, varnish, and enamel stains quickly, since a dried or hardened paint stain is almost impossible to remove. Scrape off as much of the paint or varnish as possible before using any remover. If the stain has hardened, apply a solvent on both sides and give time for it to soften. Do not rub too hard; rubbing roughens the cloth. Use one of the following methods:
Soap and water.—If the material is washable, remove fresh stains by washing with plenty of soap. If the stain has dried, soften it first by rubbing oil, lard, or petroleum jelly into it.
Turpentine or other solvents.—Sponge the stain with pure turpentine or, if the spots are large or scattered, wash the whole article in it. Or soak in a liquid of equal parts ammonia water and turpentine, rinse several times in fresh turpentine, wash in soapy water. Carbon tetrachloride, kerosene, alcohol, or benzene may be applied in the same way as turpentine. Benzene is good for the usual type of spar varnish. Alcohol will remove stains of shellac varnish, but never use alcohol on acetate rayon or vinyon.
Paint and varnish remover.—Equal parts of benzene, carbon tetrachloride, and amyl acetate (banana oil) make a very good paint remover. Apply the remover and rub in a dry-cleaning soap to help loosen the stain. Finally rinse out with carbon tetrachloride.
Do not use water on indelible pencil marks as this spreads the dye and makes the stain harder to remove. Use one of the following:
Denatured alcohol.—Soak the stain in alcohol. If carbon marks remain, sponge with soap and water. Do not use alcohol on acetate rayon. Test all dyed cloth for colorfastness.
Bleaches.—Remove the dye with a bleaching agent.
Javelle water.—For stains on uncolored cotton, linen, or rayon, dip in Javelle water for 1 minute (no longer), remove the chlorine from the cloth with a sodium thiosulfate solution, rinse well in water. (See p. 6 for more detailed instructions.) Do not use Javelle water on silk or wool.
Hydrogen peroxide and sodium perborate.—For other materials sponge with a mixture of 1 teaspoon sodium perborate to 1 pint peroxide. Rinse well.
A soft eraser sometimes will remove the marks, especially on stiff or starched materials. If the material is washable, rub soapsuds into the stain and launder as usual. Sponge woolen materials with clear water or with a solution of equal parts alcohol and water.
Perspiration of the body is usually acid, so you can sometimes restore colors changed by a perspiration stain by treating with an alkali. Dampen the stain with water and hold it over the fumes from an open ammonia water bottle. (See Acids, p. 10.) Old stains may be alkaline; then try vinegar. (See Alkalies, p. 11.) However, colors changed by perspiration cannot always be restored, particularly if the stain is an old one.
To remove perspiration odors, sponge the stained part with warm water to which a few drops of vinegar have been added, sprinkle powdered pepsin over the stain, work it well into the cloth, and let stand 1 to 2 hours, keeping the spot moist. Then brush off the powder and rinse well.
Yellowish perspiration stains on white material can be removed by:
Soap and water.—If the material is washable, bleach in the sun after washing in soap and water.
Bleaches.—For a stubborn stain, try a bleach.
Hydrogen peroxide.—Sponge with hydrogen peroxide or a mixture of 1 teaspoon sodium perborate to 1 pint peroxide. Rinse with water.
Sodium hydrosulfite.—Quickly dip the stain into a sodium hydrosulfite solution (2 teaspoons sodium hydrosulfite to 1 pint water). Rinse immediately. First test the colorfastness of the cloth to this bleach.
To remove rubber cement either sponge or dip the cloth in carbon tetrachloride, Stoddard solvent, gasoline, or benzene. If the stain has dried, rub in petroleum jelly or dry-cleaning soap to loosen it. Then apply the carbon tetrachloride or other solvent.
The acid of the vinegar or lemon juice in salad dressings may injure the color of the material. Apply a mild alkali such as baking soda or weak ammonia water immediately to restore the color. (See Acids, p. 10.) Then use one of the following to remove the stain:
Soap and water.—Sponge delicate, washable materials with lukewarm water. Do not use hot water if egg or cream was used in making the salad dressing. Use soap if it will not harm the cloth.
Grease solvents.—Sponge the stain with lukewarm water, let dry, and then sponge with carbon tetrachloride, Stoddard solvent, gasoline, or benzene.
Absorbents.—Absorbent powders are particularly good for splatters of salad oil. Dust cornstarch or talcum powder over the spot, allow it to absorb the oil or grease, then brush off. Or make a thick paste by mixing the powder with carbon tetrachloride or other grease solvent, spread it on the spot, let dry, and brush off. Repeat if necessary. Another method is to put the stained cloth between cleansing tissues and press with a warm iron.
You can usually remove light scorch stains from cotton and linen materials, but wool and silk can seldom be restored to their original condition. Brushing with emery paper may improve wool, however. Try the following:
Soap and water.—If the cloth is washable, soap and water will remove very slight stains. After washing, place the article in the sun for a day or two; it may bleach out any remaining traces of the stain.
Hydrogen peroxide.—If the stained material is white, use hydrogen peroxide. Dampen a white cotton cloth with the peroxide and lay it on the stain. Cover with a clean dry cloth; then press with a medium warm iron. If the hydrogen peroxide soaks through the top cloth, replace with a dry one. Ironing directly on the cloth moistened with peroxide or on the dampened stain itself, after the cloth has been removed, will cause rust stains on the garment. Repeat the treatment, until the stain is completely removed. Rinse well.
Light scorch stains may be removed also by sponging with hydrogen peroxide to which sodium perborate has been added (1 teaspoon sodium perborate to 1 pint peroxide). Rinse well with water.
Soap and water.—If the material is washable, remove fresh stains from one of the paste dressings by sponging or washing thoroughly with plenty of soap. For spots caused by white dressings, sponge first with water, then with soap and water.
Solvents.—Sponge well with carbon tetrachloride or turpentine. Glycerine, lard, or petroleum jelly worked into the stain first helps to loosen it. For liquid dressings and for stains on wool, sponge with denatured alcohol. Do not use alcohol on acetate rayon or colored cloth.
Bleaches.—If a dye stain remains, remove with a bleaching agent.
Hydrosulfite.—Apply one of the hydrosulfites available at drug stores as a color remover. Follow directions on the package.
Hydrogen peroxide or sodium perborate.—Sponge the stain with hydrogen peroxide or with a sodium perborate solution (p. 7) or sprinkle sodium perborate powder on the moistened stain directly. Rinse well.
Ironing material from which the soap has not been well rinsed may cause a stain much like iron rust. Washing with soap and water usually removes it. Be sure to rinse well. Bleaching in the sun afterwards is sometimes helpful.
Absorbents with solvents.—First brush the stain; then sprinkle with an absorbent powder—French chalk, cornstarch, corn meal, or salt. Work the 27 powder around until soiled and brush it off. Then if the material is washable, sponge or wash with soap and water. If water harms the cloth, first use an absorbent; then sponge the stain with one of the grease solvents—carbon tetrachloride, Stoddard solvent, or gasoline.
Another method is to make a paste by mixing an absorbent powder with carbon tetrachloride or other solvent, spread it on the stain, then brush it off when dry.
To remove the odor of smoke from a garment, have it dry-cleaned.
If the material is washable, wash out sugar-sirup stains with soap and water. For more delicate materials, sponge with clean water.
Stains made by tarlike substances are hard to remove, especially from cotton material. First rub in petroleum jelly or lard to soften the stain, then sponge with one of the grease solvents—carbon tetrachloride, Stoddard solvent, gasoline, benzene—or dip the article in the liquid and rub lightly between the hands. Repeat the treatment until the stain is removed. If the material is washable, use warm soapy water after rubbing in the petroleum jelly or lard.
For stains on carpets or rugs, scrape off as much as possible with a dull knife. Then sponge with the grease solvent, using a brushing motion so that you do not rub the stain into the carpet.
Treat stains from the tarry substances in the stem of a pipe in the same way as tar. Use one of the following methods to remove tobacco juice stains:
Cold water and glycerine.—Sponge with cold water; then work warm glycerine into the stain. Let stand for half an hour, and wash with soap and water. If the stain cannot be completely removed by washing, bleach it in the sun. Moistening it with lemon juice makes it disappear more quickly.
Wood or denatured alcohol.—To remove traces of color remaining on wool materials after the above treatment, sponge with alcohol.
Bleaches.—Try one of the following to remove remaining tobacco stains:
Hydrogen peroxide or sodium perborate.—Sponge with hydrogen peroxide or with sodium perborate solution (4 tablespoons to a pint of water). Or sprinkle powdered sodium perborate on the moistened stain. Rinse thoroughly.
Javelle water.—For stains on cotton or linen, dip the stain in Javelle water for 1 minute (no longer), remove the chlorine from the cloth with a sodium thiosulfate solution, rinse well in water. (See p. 6 for more detailed instructions.) Do not use Javelle water on silk or wool materials. Be sure to test the cloth for colorfastness before applying this bleach.
Cold water and glycerine.—Sponge the stain thoroughly with cold water to remove all the loose foodstuff. Next work glycerine into the stain, and let stand for half an hour. Then wash with soap and water.
Hydrogen peroxide or sodium perborate.—Remove any remaining stain by sponging with hydrogen peroxide or with sodium perborate solution (4 tablespoons to 1 pint of water). Sponge or rinse with cold water.
These stains differ so in composition that it is impossible to give methods which will be successful in all cases. If the color of the cloth is not destroyed but only changed, it may be restored. Normal human urine is usually acid, as is also that of all meat-eating animals. Therefore sponge such stains with a weak ammonia or soda solution. (See Acids, p. 10.) If the stain is alkaline, sponge with lemon juice or vinegar. (See Alkalies, p. 11.)
Warm water followed by salt and water.—Sponge with warm water. Warm salt water may be used (about ½ cup salt to 1 quart water). Apply and let stand 15 minutes; then sponge with clear water.
Hydrogen peroxide or sodium perborate.—Apply a few drops of hydrogen peroxide (see p. 7) or sponge with a mixture of 1 level teaspoon sodium perborate to 1 pint peroxide. Powdered sodium perborate may be sprinkled on the dampened stain. Rinse thoroughly in water.
Soap solution.—Boil washable materials in soapy water (a half-inch cube of laundry soap to each cup of water). This will completely remove fresh stains on cotton or linen. If this treatment leaves a gray color, as it sometimes does with an old stain, treat with Javelle water as follows:
Javelle water.—Mix Javelle water with an equal amount of hot water. Soak the stained place for 1½ hours in this solution, and rinse thoroughly. Then treat with oxalic acid solution (1 teaspoon oxalic acid to 1 pint water), and rinse again. This will remove a week-old stain and will not seriously injure the material. Soaking the stain in Javelle water of full strength, however, rots the material. Do not use Javelle water on silk or wool.
Some silks, rayons, and wools are spotted by water. To remove such spots, dampen the entire material evenly, either by sponging with clean water or by shaking in the steam from a briskly boiling teakettle. Then press it while still damp. Scratching with the fingernail or a stiff brush or rubbing the cloth between the hands will sometimes remove the spot.
If the material is washable, laundering in warm soap and water will remove these spots. For nonwashable materials, sponge with warm water, let dry, then sponge with a grease solvent—carbon tetrachloride, gasoline, or benzene.
A B C D E F G H I J K L M N O P Q R S T U V W X Y Z
* U. S. GOVERNMENT PRINTING OFFICE: 1955 O-337590
For sale by the Superintendent of Documents, U. S. Government Printing Office
Washington 25, D. C.—Price 15 cents